How do Broiler Breeders and In-ovo Feeding Impact on Chick Quality?
Sandro Cerrate, PhD
Credinser LLC, Madison, Alabama
Published: April 6, 2020
Sometimes chicken flocks resulted in less livability and body weight at seven days of age than other ones without knowing the reason. Besides, this reduced performance at an early age has a carryover effect until the market age. What was the cause? Probably, the lower broiler performance is mostly related to the quality of the chicks that arrive at the farms. Indeed, the broiler breeders, incubation process, or in-ovo feeding impact on chick quality.
The eggshell temperature, brooding temperature, and additives may influence also the chicken growth at an early age, as described in Pasty Vents in Broiler Chickens. Poultry managers should identify those variables to avoid bias in chick quality analysis. For example, if the mortality is higher than 1.0% at seven days and brooding management was excellently managed, likely the incubation process or broiler breeders provoked this diminishing chick performance. The strong relationship of body weights between seven days and market age indicates that the importance of chick quality and his carryover effects.
To excel at the chick quality, the (a) breeder management, (b) dietary breeder nutrients, and (c) in-ovo feeding are analyzed.
a) Breeder management
The breeder management implies different aspects, which are directly related to the rearing of birds, such as stress factors, feeding allocation, and egg management (Figure 1). Maternal stress conditions increased the egg corticosterone levels, impairing the hatchability and offspring’s live weight in barn swallows (Saino et al. 2005). These findings remain feasible as a concept and open opportunities to run stress conditions in broiler breeders.
Figure 1. Effect of stress, microcrack, overweight, and dirty eggs on progeny chickens.
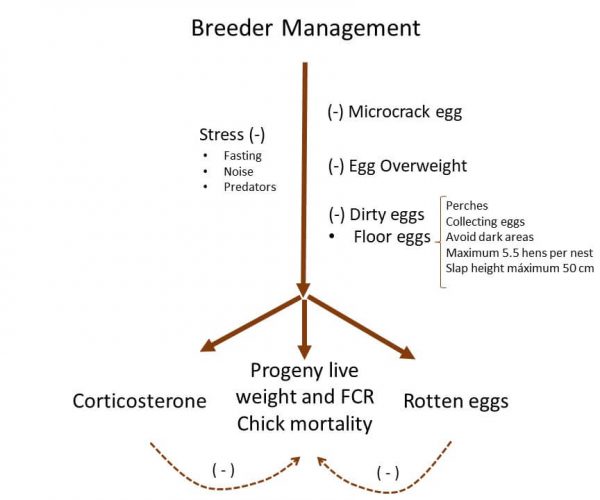
Note: (-) negative responses on progeny live performance.
Fasting period, noise, or predators can be considered stress factors that may trigger the levels of corticosterone. To demonstrate, enlarging the length of the fasting period for pullets has also elevated the corticosterone levels in mature broiler breeders (Ekmay et al. 2010). The latter fact might induce a carryover effect during the production phase and progeny live performance. Even more, broiler breeder hens fed two or three times per day generated better progeny feed conversion and body weight as compared to one meal per day (Gholami et al. 2017).
Egg management is crucial to avoid crack, floor, and dirty eggs. Microcrack eggs and egg overweight by facilitating the egg contamination cause a high incidence of rotten eggs during the incubation. These rotten eggs increase more as eggs become dirty allowing bacterial penetration, embryonic, and chick mortality. As expected, rotten eggs during the incubation produced higher embryonic mortality (van den Brand et al. 2016) and chick mortality (Barnett et al. 2004). For this reason, cleaning dirty eggs (Yoho et al. 2008) and avoiding floor eggs before setting in the incubation will reduce the incidence of rotten eggs.
To avoid floor eggs, Valle (2008) suggested placing perches in the rear period, stimulating jumping, and nesting behavior. Besides, the following techniques may reduce the possibility of non-nest eggs as collecting the floor eggs frequently, avoiding dark areas in a lay house, the timing of feeding and lay should not match, setting up to 5.5 hens per nest, and nest or slat height up to 50 cm.
b) Dietary breeder nutrients
The type of nutrients and feeding of broiler breeders notably affect the progeny performance. Diets for roosters may include a wide range of antioxidant nutrients, that along with proper male bodyweight management, might promote the semen quality and concomitantly the progeny performance (Figure 2). As compared to female diets, these enriched male diets will be cheaper in chick output per nutrient intake and producing a similar positive impact on chick quality. On the other hand, hen diets that include fiber, proper amino acid balance, antioxidants, nutrients for egg mineralization, and antimicrobials will control the egg overweight or egg rotten and finally will enhance the chick quality.
Figure 2. Effect of rooster and hen diets on progeny live performance.
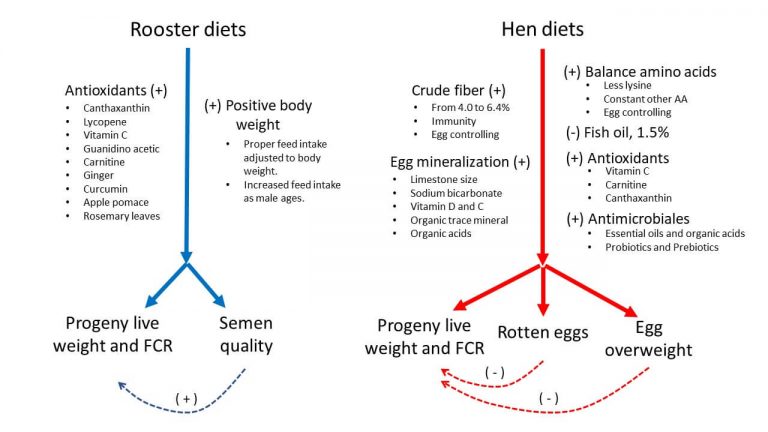
Note: (+) positive response and (-) negative response on progeny live performance.
Rooster diets
Roosters fed antioxidants stimulate the chicken quality along with growing antioxidant properties and fertility. Male older breeders providing antioxidants improved markedly the testes weights, progeny body weight by 70 grams, and feed conversion by 0.10 or 10 points (Triques et al. 2019). These given antioxidants consisted of 8 ppm of canthaxanthin, 40 ppm of lycopene, and 150 ppm of vitamin C. Moreover, male breeders fed 500 ppm of vitamin C (Khan et al. 2013) and 0.5 g of lycopene per liter of water (Mangiagalli et al. 2010), increased the semen quality and fertility by 10%, respectively. In the same way, roosters fed several additives enhanced the semen antioxidant capacity and fertility. These metabolites were guanidino acetic acid, carnitine, ginger, curcumin, apple pomace, and rosemary leaves (Akhlaghi et al. 2014a,b; Tapeh et al. 2017; Borghei-Rad et al. 2017; Kazemizadeh et al. 2019). Inferring, this antioxidant capacity not only protects the semen polyunsaturated fatty acids but also transfers their chemical components into the eggs.
At the same time, it is essential to keep a positive body weight gain as male ages for avoiding a seminal fatty acid damage, and thereby enhancing the fertility and chick quality. Animals in a fasting state have reduced the polyunsaturated fatty acids in the testes (Dayangac et al. 2011). As a result, any reduction in the testes weight due to decreasing feed intake or body weight gain will affect the semen quality. For this reason, it is crucial to calculate the feed intake accurately, by using algorithms as described in How to Improve the Fertility and Hatchability in Broiler Breeders. In particular, as compared to constant feed intake during the lay period, male breeders fed escalating feed intake from 100 to 124 grams per day earned better progeny body weight by 140 g and feed conversion by 3 points at 42 days (Romero-Sanchez, 2005).
Macronutrients in hen diets
Hens fed macronutrients as fiber, protein, amino acids, and oils displayed a carryover effect on progeny performance. Notably, broiler breeder hens fed more dietary crude fiber from 4.0% to 6.4% rose notably the offspring live weight and livability (Enting et al. 2007) by controlling the egg size and possibly enhancing the immunity. Another way for decreasing large egg size and fortifying chick quality is by adjusting the amino acids, in particular the digestible lysine. To illustrate, hens receiving balance amino acids and lessened protein and lysine in the laying period boosted the progeny live performance. For example, female broiler breeders fed less digestible lysine intake between 700 and 1000 grams, but keeping constant all other amino acids, enhanced the progeny feed conversion (Ciacciariello and Tyler, 2013) in 5 points at market age (Mejia et al. 2013).
Conversely, field information suggests that pullets fed smaller amino acid intakes than needed developing less fertile eggs at late production, and less progeny livability. Also, feeding fish oil to breeder hens might affect chick quality negatively. As an illustration, breeder hens fed 1.5% of fish oil affected the progeny live weight and feed conversion negatively (Koppenol et al. 2015). The high levels of docosapentaenoic and docosahexaenoic in hens fed fish oil may shift the fat metabolism, transferring the fatty acids from the yellow follicles to the abdominal fat, and also slowing down the synthesis of albumen.
It is a common misunderstanding that large eggs derive in big chickens. Ulmer-Franco et al. (2010) demonstrated, for instance, that large eggs did not yield extra progeny body weight at market age. On the other hand, if the eggs are smaller than recommended for a particular hen age, the progeny body weights at market age will be diminished by 120 g at 29 weeks of age, and by 40 g at 59 weeks of age (Ulmer-Franco et al. 2010). Hence, a proper egg weight profile will result in better progeny chick quality.
Hen nutrients on egg mineralization
Limestone size, mineral, vitamins, and additives in hen diets do affect the egg mineralization, thus influencing the rotten eggs and chick quality. Calcium and vitamin D metabolism is critical for improving the eggshell quality. Accordingly, hens need two sizes of limestone as 20% of fine (0.5-2.0mm) and 80% of coarse (2-4 mm) for supplying calcium during the day and night during eggshell formation respectively. This mixture of limestone sizes will increase the egg specific gravity and reduce the hairline crack eggs.
Calcium digestibility (Scheideler, 1998) and the enzymatic conversion for calcitriol (Abe et al. 1982) impacted negatively as hens age. For this reason, hens fed calcitriol decline the incidence of egg breakage (Tsang et al. 1992) by facilitating the deposition of calcium during egg formation. Furthermore, hens fed sodium bicarbonate, vitamin C, and organic trace minerals fortified the eggshell quality (Frank and Burger, 1965; Manangi et al. 2015; Chung et al. 2005), consequently diminishing the incidence of hairline eggs.
Additives lessening the dirty and cracked eggs will also support chick quality. To illustrate, dirty and crack eggs diminished in half when older breeder hens fed dietary short fatty acids or organic acids (Sengor et al. 2007). This positive response might be explained by the higher retention of calcium and phosphorous when the organic acids acidified the intestinal digesta (Youssef et al. 2013; Khong et al. 2014).
Hen nutrients on antioxidant
Hens providing antioxidants increase the egg antioxidants and stimulate probably the chick quality. In the same way, hens fed 6 ppm of canthaxanthin enlarged the antioxidant properties in eggs (Rosa et al. 2012). In this study, the progeny performance was not measured. Nevertheless, broilers from in-ovo feeding of canthaxanthin at 0.05 mg/egg grew better at 28 days as compared to those from the water injected group (Ismail et al. 2019). Similarly, breeder hens fed a combination between canthaxanthin and 25OHD3 yielded better progeny feed conversion and breast meat yield at 21 days of age (Araujo et al. 2019). The latter variable was rose better as an additive effect when both hens and chickens fed this combination.
Hen nutrients on antimicrobials
Older literature suggests that hens fed antibiotics benefit the progeny bodyweight satisfactorily (Bentley and Hershberger, 1954). However, modern studies are missing about the impacts of broiler breeders given antibiotics on progeny performance in comparison with new alternatives. In contrast, current progeny studies using alternatives for antibiotics have been tested for potential replacement of antibiotics, yet without comparing with antibiotics.
For instance, female broiler breeders fed combination between essential oils and organic acids in corn-soybean based diets enriched the progeny body weight in 485 grams, feed conversion in 16 points, and humoral immunity (Toosi et al. 2016). In this study, eggs injected with this combination indicated a similar trend, though with fewer degrees. Broiler chickens from this in-ovo combination grew, for example, heavier by 153 grams and converted better the feed by 13 points as compared to those from a water in-ovo injection.
The inclusion of Bacillus subtilis in breeder hen diets generated better offspring body weight in 236 grams and feed conversion in 10 points at 56 days old as compared to those from hens fed without this probiotic (Owen, 2017). Interestingly, broiler chickens fed this probiotic, without hens fed this additive, showed similar advantages in terms of body weight and feed conversion as those chickens from hens fed the Bacillus subtilis. This striking information reveals that not only the probiotics will offer advantages to broiler chickens directly, but also hens fed this additive will yield exponential gains over progeny broiler chickens, considering that one hen equates around 145 chicks.
Female broiler breeders feeding β -glucans and mannan-oligosaccharides derived from yeasts presented better progeny body weight, feed conversion (Araujo et al. 2018), and breast muscle meat (Kidd et al. 2013). The progeny advantages from this prebiotics seem to increase as breeder hens grew older.
c) In-ovo feeding
In the last years, a growing number of in-ovo feeding studies reported positive events on progeny performance, immunity, breast muscle yield, and intestinal growth (Figure 3). This type of research opens fields for further studies either during the broiler breeder diets or during the in-ovo feeding. Principally, do these nutrients producing positive responses from in-ovo feeding might imply a certain degree of hen nutrient deficiencies? If they do illustrate maternal nutrient deficiencies, could those nutrients be also added to hen diets without affecting the breeder performance? To analyze the significance of the in-ovo treatments, the author selected only the trials that included the in-ovo saline or water group for comparative purposes.
Figure 3. In-ovo feeding on progeny live performance.
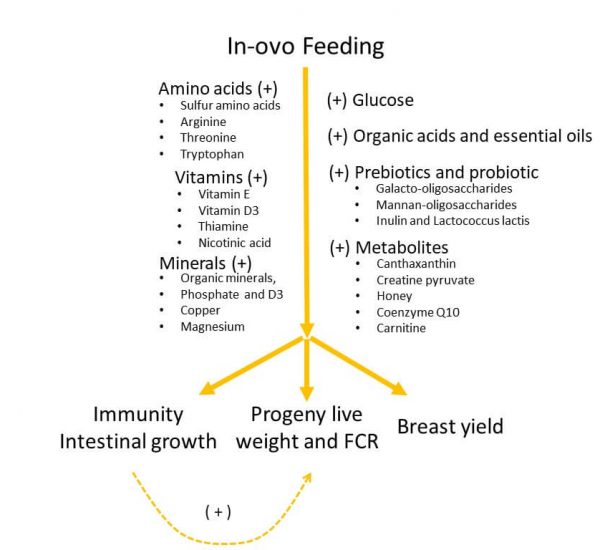
Note: (+) positive responses on progeny live performance.
Glucose
The use of glucose in ovo (100 mg/egg) produces better body weight by 110 grams and feed conversion by 8 points as compared to those from the control group (Salmanzadeh et al. 2012). When eggs received in both the glucose and magnesium (4 mg/egg), the broilers grew better in 218 grams, converted more the feed by 15 points, and deposited more the breast meat yield in 1.1%. According to these authors, glucose and magnesium support the energy status elevating the glycogen reserves and saving the muscle protein for the post-hatch growth.
Amino acids
The in-ovo feeding for methionine, cysteine, and lysine shows uneven responses on broiler performance. In-ovo technique of methionine+cysteine strengthened, for instance, the progeny antioxidant capacity (Elwan et al. 2019; Elnesr et al. 2019); nonetheless, in-ovo feeding of either lysine or methionine did not affect the body weight and feed conversion (Coskun et al. 2019). Compared to those amino acids, in-ovo feeding for threonine, arginine, and tryptophan reveals consistent outcomes. The in-ovo solutions with the only arginine without lysine resulted in more than 100 grams of body weight at 35 days as compared to those from eggs injected with saline water (Shafey et al. 2014).
Additionally, in another research, chickens from eggs injected with arginine at 17 days of incubation increased the breast muscle yield and breast amino acid concentration at 21 days of age (Yu et al. 2018). Under other conditions, adding threonine through the yolk or the amniotic fluid fortified the immunity (Kadam et al. 2008), villus growth at 21 d (Moreira-Filho et al. 2019), body weight, and feed conversion at 42d (Toghyani et al. 2019). Another in-ovo amino acid depicting great results is tryptophan. This amino acid enhanced the progeny breast meat yield (33.1% vs 31.1%) and livability at 35 days (Nayak et al. 2018).
Therefore, in general, these current studies indicate that feeding in-ovo arginine, threonine, and tryptophan will be beneficial on the chicken performance, and adding in-ovo methionine+cysteine may benefit the antioxidant capacity.
Vitamins
The studies of in-ovo feeding with vitamins are extensive and overwhelming. Chicks from eggs injected with 60 IU of vitamin E hatched more from 86% to 92% and grew faster the duodenum villus at hatch (Araujo et al. 2019). In this study, the addition of vitamin E up to 27.5 IU developed better feed conversion at 21 days of age. Similarly, eggs injected at 2.4 ug of 25 OHD3 derived in better feed conversion by 7 points at 14 days (Fatemi et al. 2019). In the same vein, chickens from in-ovo feeding of thiamine (B1) or nicotinic acid (B3) grew faster at 28 days and market age, respectively (Bhanja et al. 2012; Parnian et al. 2019).
Contrarily, in ovo-feeding of several vitamins did not affect the broiler performance. These vitamins are vitamin A, vitamin C, riboflavin (B2), folic acid, pantothenic acid, pyridoxine (B6), cyanocobalamin (B12), betaine, and choline (Glodek et al. 2010; Bhanja et al. 2012; Gholami et al. 2015; Momeneh and Torki, 2018; Parnian et al. 2019; Zhang et al. 2019).
Minerals
In-ovo combination of minerals and vitamins showed positive outcomes on broiler chicken performance. Organic trace minerals as glycine chelates, phosphate, and vitamin D3 injected in eggs strengthened the bone ash content at 38 days of age (Yair et al. 2015). Moreover, in-ovo feeding of different copper sources as sulfate, acetate, and nanoparticles boosted the chick weight at hatch (Arafat et al. 2019).
Prebiotics
Consistently, chickens from eggs injected with galacto-oligosaccharides grew heavier by 80-100 grams at market age as compared to those from eggs with saline solution (Pruszynska-Oszmalek et al. 2015; Dankowiakowska et al. 2019; Slawinska et al. 2020). Furthermore, in-ovo feeding of mannan-oligosaccharide enlarged the villus height and jejunum area at hatch (Cheled-Shoval et al. 2011). In contrast, in-ovo feeding of raffinose and inulin displayed similar broiler performance as those from the saline group (Maiorano et al. 2012; Pruszynska-Oszmalek et al. 2015).
Probiotics
Chickens from an in-ovo combination between inulin and Lactococcus lactis grew faster (Pruszynska-Oszmalek et al. 2015). However, in-ovo feeding of Lactobacillus animalis, Enterococcus faecium (Beck et al. 2019), and a combination of Lactobacillus salivarius and galacto-oligosaccharides (Tavaniello et al. 2019) did not affect the body weight, feed conversion, and carcass yield.
Metabolites
Fertile eggs injected with 6 mg or 12 mg of creatine pyruvate enlarged the chick body weight (~6%) and breast muscle yield at 21 days as compared to the saline group (Zhao et al. 2017). The creatine as an energy reserve in the chicks might conserve the protein reserve in the muscle and use for posthatch protein growth. Carnitine, a metabolite responsible for the fatty acid transportation into the mitochondria, promises as an alternative to be used as in-ovo feeding. As an example, the injection of L-carnitine at 32 mg/100 uL boosted the feed conversion in 4 points at 45 days of age (Dooley et al. 2011). Herein, compared to this in-ovo L-carnitine, the extra addition in broiler of dietary L-carnitine (50 ppm) from in-ovo L-carnitine eggs yielded similar results on broiler performance.
The application of honey in ovo at 17 days into yolk sac stimulated greatly the feed conversion by 20 points, body weight by 178 grams, and intestinal villi height by 19% at 42 days of age (Abdullah et al. 2018). These excellent benefits of honey might be explained due to his antibacterial and antioxidant properties (Alzahrani et al. 2012) which might depend on his polyphenols and organic acids (Mato et al. 2003; Alzahrani et al. 2012), being tartaric acid the highest among the organic acids (Keke and Cinkmanis, 2019). For instance, in-ovo feeding of honey modified the caecal microflora reducing Salmonella and Escherichia coli and increasing Lactobacilli positively (Memon et al. 2019).
Coenzyme Q10 injected in ovo at 0.2 ml per egg improved the immunity system, body weight by 310 grams, and feed conversion by 14 points (Kalantar et al. 2019). In this study, this additive, which is essential in the formation of ATP, also elevated the hatchability and chick weight at hatch.
Nutrient enrichment
Could these in-ovo nutrients, as shown in Figure 3, be fed in broiler breeders above their requirements and induce depositions into the eggs? Indeed, several trace minerals and vitamins (Table 1) augmented their concentrations into the eggs when hens fed dietary nutrient levels generally above their requirements.
On the contrary, few dietary trace minerals and vitamins did not respond linearly on their deposition in eggs. Particularly, when hens fed copper from 9 to 20 ppm or manganese from 60 to 80 ppm, the copper or manganese in egg plateaued (Berwanger et al. 2018; Li et al. 2018). In agreement with this finding, Nober et al., (1979) proposed that enrichment of dietary amino acids, ascorbic acid, calcium, phosphorous, sodium, potassium, chloride, magnesium, and copper produce little or no variation in eggs. In contrast to those suggestions, vitamins in the eggs responded linearly by adding nutrients above their requirements, except for pyridoxine. To illustrate, hens fed pyridoxine above 3 mg/kg plateaued up to 5 mg/kg (Fuller et al. 1961).
Table 1. Hen nutrient levels that influence their nutrient deposition in eggs.
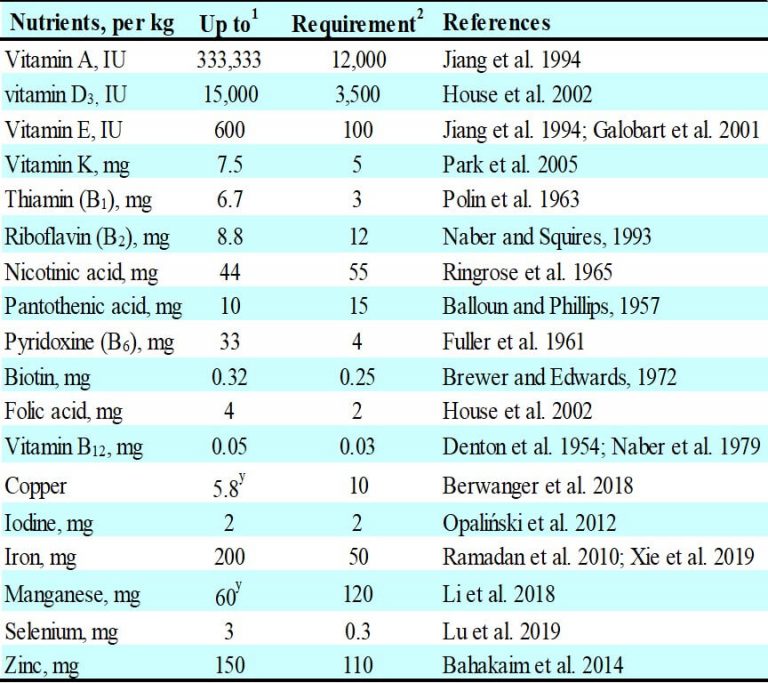
Note: 1Up to those levels of dietary nutrients increased linearly their egg nutrient concentration. 2Requirement from a primary breeding company. yAbove those dietary levels their egg nutrient contents were constant.
Conclusions:
- Progeny live performance and chick mortality are affected by rotten eggs and corticosterone of broiler breeders. Thus, stress conditions elevate egg corticosterone, while egg management as microcrack, overweight, and dirty eggs influences the rotten eggs.
- Positive body weight gain and dietary antioxidants in roosters promote the semen quality, progeny live weight, and offspring feed conversion. Hen dietary macronutrients, minerals, vitamins, antioxidants, and antimicrobials yield better progeny performance by controlling the egg weight and rotten eggs, whereas fish oil reduces the chick quality.
- In-ovo feeding of glucose, amino acids, minerals, vitamins, organic acids, essential oils, prebiotics, probiotics, and metabolites promote the broiler performance. On the other hand, enrichment of some dietary breeder vitamins and trace minerals might influence the egg content and chick quality.
The author declares that this article was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Every attempt has been made to ensure that the material in this article is accurate, true, correct, and relevant at the time of writing. However, the author accepts no liability for any omissions, damage, loss, or financial consequences of using this article.
Abdullah, S., I. H Leghari, A. A. Moriani, N. Rajput, J. A. Gandhai, and M. Nisa. 2018. Effect of in ovo supplementation of honey in fertile eggs on posthatch growth performance of broiler chickens. J. Anim. Plant Sci. 28:1584-1590.
Abe, E., H. Horikawa, T. Masumura, M. Sugahara, M. Kubota, and T. Suda. 1982. Disorders of cholecaiciferol metabolism in old egg-laying hens. J. Nutr. 112:436-446.
Akhlaghi, A., Y. Jafari Ahangari, B. Navidshad, Z. Ansari Pirsaraei, M. Zhandi, H. Deldar, M. R. Rezvani, M. Dadpasand, S. R. Hashemi, R. Poureslami, and E. D. Peebles. 2014a. Improvements in semen quality, sperm fatty acids, and reproductive performance in aged Cobb 500 breeder roosters fed diets containing dried ginger rhizomes (Zingiber officinale). Poult. Sci. 93:1236-1244.
Akhlaghi, A., Y. Jafari Ahangari, M. Zhandi, and E. D. Peebles. 2014b. Reproductive performance, semen quality, and fatty acid profile of spermatozoa in senescent broiler breeder roosters as enhanced by the long-term feeding of dried apple pomace. Anim. Reprod. Sci. 147:64-73.
Alzahrani, H. A., R. Alsabehi, L. Boukraa, F. Abdellah, Y. Bellik, and B. A. Bakhotmah. 2012. Antibacterial and antioxidant potency of floral honeys from different botanical and geographical origins. Molecules 17:10540-10549.
Arafat, A. R., H. A. Hassan, K. Y. Farroh, S. S. Elnesr, I. EL-wardany, and M. S. Bahnas. 2019. Effects of copper (sulfate, acetate and nano) in ovo injection on hatching traits and some physiological parameters of newly-hatched broiler chicks. J. Adv. Lab. Res. Biol. 10:65-72.
Araujo, I., M. B. Café, R. A. Noleto, J. Martins, C. J. Ulhoa, G. C. Guareshi, M. M. Reis, and N. S. Leandro. 2018. Effect of vitamin E in ovo feeding to broiler embryos on hatchability, chick quality, oxidative state, and performance. Poult. Sci. 98:3652-3661.
Araujo, L. F., C. S. S. Araujo, R. J. G. Pereira, L. C. Bittencourt, C. C., Silva, F. Cisneros, R. G. Hermes, Y. G. A. Sartore, and M. T. Dias. 2019. The dietary supplementation of canthaxanthin in combination with 25OHD3 results in reproductive, performance, and progeny quality gains in broiler breeders. Poult. Sci. 98:5801-5808.
Araujo, L. F., M. Bonato, R. Barbalho, C.S. D. S. Araujo, P. S., Zorzetto, C. A. Granghelli, R. J. G. Pereira, and A. j. T. Kawaoku. 2018. Evaluating hydrolyzed yeast in the diet of broiler breeder hens. J. Appl. Poult. Res. 27:65-70.
Bahakaim, A. S. A., and H. A. Abdel, S. M. H. Osman, A. S. Omar, N. Y. A. Malak, and N. A. Ramadan. 2014. Effect of using different levels and sources of zinc in layer’s diets on egg zinc enrichment. Egyptian Poultry Science Journal. 34:39-56.
Balloun, S. L., and R. E. Phillips. 1957. Interaction effects of vitamin B12 and pantothenic acid in breeder hen diets on hatchability, chick growth and livability. Poult. Sci. 36:929-934.
Barnett, D. M., B. L. Kumpula, R. L. Petryk, N. A. Robinson, R. A. Renema, and F. E. Robinson. 2004. Hatchability and early chick growth potential of broiler breeder eggs with hairline cracks. J. Appl. Poult. Res. 13:65-70.
Beck, C. N., C. D. McDaniel, K. GS Wamsley, and A.S. Kiess. 2019. The potential for inoculating Lactobacillus animalis and Enterococcus faecium alone or in combination using commercial in ovo technology without negatively impacting hatch and post-hatch performance. Poult. Sci. 98:7050-7062.
Bentley, O. G., and T. V. Hershberger. 1954. The Effect of Antibiotics on Hatchability of Hens’ Eggs and Progeny Growth Performance. Poult. Sci. 33:641-648.
Berwanger, E., S. L. Vieira, C. R. Angel, L. Kindlein, A. N. Mayer, M. A. Ebbing, and M. Lopes. 2018. Copper requirements of broiler breeder hens. Poult. Sci. 97:2785-2797.
Bhanja, S. K., A. B. Mandal, S. Majumdar, M. Mehra, and A. Goel. 2012. Effect of in ovo injection of vitamins on the chick weight and post-hatch growth performance in broiler chickens. Indian Journal of Poultry Science. 47:306-310.
Borghei-Rad, S. M., S. Zeinoaldini, M. Zhandi, H. Moravej, and M. Ansari. 2017. Feeding rosemary leaves powder ameliorates rooster age-related subfertility. Theriogenology 101:35-43.
Brewer, L. E., and H. M. Edwards Jr. 1972. Studies on the biotin requirement of broiler breeders. Poult. Sci. 51:619-624.
Cheled-Shoval, S. L., E. Amit-Romach, M. Barbakov, and Z. Uni. 2011. The effect of in ovo administration of mannan oligosaccharide on small intestine development during the pre-and posthatch periods in chickens. Poult. Sci. 90:2301-2310.
Chung, M. K., J. H. Choi, Y. K. Chung, and K. M. Chee. 2005. Effects of dietary vitamins C and E on egg shell quality of broiler breeder hens exposed to heat stress. Asian Australas. J. Anim. Sci. 18:545-551.
Ciacciariello, M., and N. C. Tyler. 2013. The effects of maternal dietary lysine intake on offspring performance to 21 days of age. J. Appl. Poultry Res. 22:238-244.
Coskun, I., A. Akkan, and G. Erener. 2018. Effects of in ovo injection of lysine and methionine into fertile broiler (parent stock) eggs on hatchability, growth performance, caecum microbiota, and ileum histomorphology. R. Bras. Zootec. 47:e20170220.
Dankowiakowska, A., J. Bogucka, A. Sobolewska, S. Tavaniello, G. Maiorano, and M. Bednarczyk. 2019. Effects of in ovo injection of prebiotics and synbiotics on the productive performance and microstructural features of the superficial pectoral muscle in broiler chickens. Poult. Sci. 98:5157-5165.
Dayangac, A., M. Bahsi, A. Ozkaya, and O. Yilmaz. 2011. Linalool improve biochemical damage and fatty acids composition of testes on fasting male rats. J. Anim. Vet. Adv. 10:1232-1238.
Denton, C. A., W. L. Kellogg, J. R. Sizemore, and R. J. Lillie. 1954. Effect of injecting and feeding vitamin B12 to hens on content of the vitamin in the egg and blood: One figure. J. Nutr. 54:571-577.
Dooley, M. R. 2011. Evaluation of L-carnitine in ovo injection followed by L-carnitine feed supplementation on broiler hatching and growing characteristics. PhD Diss. Mississippi State Univ.
Ekmay, R. D., M. De Beer, R. W. Rosebrough, M. P. Richards, J. P. McMurtry, and C. N. Coon. 2010. The role of feeding regimens in regulating metabolism of sexually mature broiler breeders. Poult. Sci. 89:1171-1181.
Elnesr, S. S., H. A. M. Elwan, Q. Q. Xu, C. Xie, X. Y. Dong, and X. T. Zou. 2019. Effects of in ovo injection of sulfur-containing amino acids on heat shock protein 70, corticosterone hormone, antioxidant indices, and lipid profile of newly hatched broiler chicks exposed to heat stress during incubation. Poult. Sci. 98:2290-2298.
Elwan, H. A., S. S. Elnesr, Q. Xu, C. Xie, X. Dong, and X. Zou. 2019. Effects of in ovo methionine-cysteine injection on embryonic development, antioxidant status, IGF-I and TLR4 gene expression, and jejunum histomorphometry in newly hatched broiler chicks exposed to heat stress during Incubation. Animals. 9:25. doi:10.3390/ani9010025
Enting, H., T. A. M. Kruip, M. W. A. Verstegen, and P. J. Van der Aar. 2007. The effect of low-density diets on broiler breeder performance during the laying period and on embryonic development of their offspring. Poult. Sci. 86:850-856.
Fatemi, S. A., K. E. C. Elliott, A. Bello, O. A. Durojaye, H. J. Zhang, and E. D. Peebles. 2019. The effects of in ovo injected vitamin D3 sources on the eggshell temperature and early posthatch performance of Ross 708 broilers. Poult. Sci. https://doi.org/10.1016/j.psj.2019.10.055
Frank, F.R., and R. E. Burger. 1965. The effect of carbon dioxide inhalation and sodium bicarbonate ingestion on egg shell deposition. Poult. Sci. 44:1604-1606.
Fuller, H. L., R. C. Field, R. Roncalli-Amici, W. S. Dunahoo, and H. M. Edwards Jr. 1961. The vitamin B6 requirement of breeder hens. Poult. Sci. 40:249-253.
Galobart, J., A. C. Barroeta, M. D. Baucells, and F. Guardiola. 2001. Lipid oxidation in fresh and spray-dried eggs enriched with ω3 and ω6 polyunsaturated fatty acids during storage as affected by dietary vitamin E and canthaxanthin supplementation. Poult. Sci. 80:327-337.
Gholami, J., A. A. Qotbi, A. Seidavi, A., Meluzzi, S. Tavaniello, and G. Maiorano. 2015. Effects of in ovo administration of betaine and choline on hatchability results, growth and carcass characteristics and immune response of broiler chickens. Italian Journal of Animal Science. 14:187-192.
Gholami, M., A. Seidavi, C. J. O’Shea, Y. Akter, M. Dadashbeiki, and B. Bahar. 2017. Feeding regimen of breeder broiler hen influences growth performance of the broiler chickens. Livest. Sci. 203:132-135.
Glodek, K., M. W. Lis, B. Płytycz, A. Mazur, and J. W. Niedziółka. 2010. Effect of high dose of riboflavin injected in ovo on hatchability and gain of broiler chicken. Anim. Biol. 12:269-275
House, J. D., K. Braun, D. M. Ballance, C. P. O’connor, and W. Guenter. 2002. The enrichment of eggs with folic acid through supplementation of the laying hen diet. Poult. Sci. 81:1332-1337.
Ismail, F. S. A., K. Sherif, Y. S. Rizk, and M. E. Hassan. 2019. Effect of spirulina and canthaxanthin injection into hatching eggs on hatchability traits and subsequent growth performance of chicks. Journal of Animal and Poultry Production. 10:197-202.
Jiang, Y. H., R. B. McGeachin, and C. A. Bailey. 1994. α-Tocopherol, β-carotene, and retinol enrichment of chicken eggs. Poult. Sci. 73:1137-1143.
Kadam MM, Bhanja SK, Mandal AB, Thakur R, Vasan P, Bhattacharyya A, and J. S. Tyagi. 2008. Effect of in ovo threonine supplementation on early growth, immunological responses and digestive enzyme activities in broiler chickens. Br. Poult. Sci. 49:736-741.
Kalantar, M., S. M. Hosseini, M. R. Hosseini, M. H. Kalantar, and L. G. Yang. 2019. Effects of in ovo injection of coenzyme Q10 on hatchability, subsequent performance, and immunity of broiler chickens. BioMed Res. Inter. https://doi.org/10.1155/2019/7167525
Kazemizadeh, A., A. Z. Shahneh, S. Zeinoaldini, A. R. Yousefi, H. M. Yeganeh, Z. A. Pirsaraei, and A. Akhlaghi. 2019. Effects of dietary Curcumin supplementation on seminal quality indices and fertility rate in broiler breeder roosters. Br. Poult. Sci. DOI: 10.1080/00071668.2019.1571165
Keke, A. and I. Cinkmanis. 2019. Determination of organic acids in honey samples from latvian market by high-performance liquid chromatography. Research for Rural Development. 1: DOI: 10.22616/rrd.25.2019.034
Khan, R. U., Z. U. Rahman, I. Javed, and F. Muhammad. 2013 Effect of vitamins, probiotics and protein level on semen traits and seminal plasma biochemical parameters of post-moult male broiler breeders. Br. Poult. Sci. 54:120-129.
Khong, C., S. Sen, S. Lee, Y. Choi, K. Y. Kim, S. Ingale, and I. K. Kwon. 2014. Effect of sodium butyrate supplementation on performance, egg quality and bacterial load in the excreta of laying hens. J. Anim. Res. 4:141-153.
Kidd, M. T., L. Araujo, C. Araujo, C. D. McDaniel, and D. McIntyre. 2013. A study assessing hen and progeny performance through dam diet fortification with a Saccharomyces cerevisiae fermentation product. J. Appl. Poult. Res. 22:872-877.
Koppenol, A., E. Delezie, Y. Wang, L. Franssens, E. Willems, B. Ampe, B, J. Buyse, and N. Everaert. 2015. Effects of maternal dietary EPA and DHA supplementation and breeder age on embryonic and post-hatch performance of broiler offspring. J. Anim. Physiol. A. Anim. Nutr. 99:36-47.
Li, L. L., N. N. Zhang, Y. J. Gong, M. Y. Zhou, H. Q. Zhan, and X. T. Zou. 2018. Effects of dietary Mn-methionine supplementation on the egg quality of laying hens. Poult. Sci. 97:247-254.
Lu, J., L. Qu, M. M. Shen, X. G. Wang, J. Guo, Y. P. Hu, T. C. Dou, and K. H. Wang. 2019. Effects of high-dose selenium-enriched yeast on laying performance, egg quality, clinical blood parameters, organ development, and selenium deposition in laying hens. Poult. Sci. 98:2522-2530.
Maiorano, G., A. Sobolewska, D. Cianciullo, K. Walasik, G. Elminowska-Wenda, A. Sławińska, S. Tavaniello, J. Zylińska, J. Bardowski, and M. Bednarczyk. 2012. Influence of in ovo prebiotic and synbiotic administration on meat quality of broiler chickens. Poult. Sci. 91:2963-2969.
Manangi, M. K., M. Vazques-Añon, J. D. Richards, S. Carter, and C. D. Knight. 2015. The impact of feeding supplemental chelated trace minerals on shell quality, tibia breaking strength, and immune response in laying hens. J. Appl. Poult. Res. 00:1-11.
Mangiagalli, M. G., P. A. Martino, T. Smajlovic, L. Guidobono Cavalchini, and S. P. Marelli. 2010. Effect of lycopene on semen quality, fertility and native immunity of broiler breeder. Br. Poult. Sci. 51:152-157.
Mato, I. S., J. F. Huidobro, J. Jesu, J. Simal-Lozano, and A. M. T. Sancho. 2003. Significance of Nonaromatic organic acids in honey. Journal of Food Protection. 66:2371-2376.
Mattila, P., J. Valaja, L. Rossow, E. Venäläinen, and T. Tupasela. 2004. Effect of vitamin D2-and D3-enriched diets on egg vitamin D content, production, and bird condition during an entire production period. Poult. Sci. 83:433-440.
Mejia, L., C. D. McDaniel, M. T. Kidd, K. Lopez, and A. Corzo. 2013. Evaluation of carryover effects of dietary lysine intake by Cobb 500 broiler breeder hens. Poult. Sci. 92:709-718.
Memon, S. S., A. A. Kamboh, I. H. Leghari, and R. A. Leghari. 2019. Effect of in ovo and post-hatch administration of honey on the immunity and intestinal microflora of growing chickens. J. Anim, Feed Sci. 28:346-353.
Momeneh, T., and M. Torki. 2018. Effects of in ovo injection of vitamins B 6 and B 12 in fertile eggs subjected to ethanol stress on hatching traits, performance and visceral organs of broiler chicks reared under cold stress condition. Iran. J. Appl. Anim. Sci. 8:491-498.
Moreira-Filho, A. L. B., P. R. Ferket, R. D. Malheiros, C. J. B. Oliveira, P. C. Aristimunha, D. E. Wilsmann, and P. E. N. Givisiez. 2018. Enrichment of the amnion with threonine in chicken embryos affects the small intestine development, ileal gene expression and performance of broilers between 1 and 21 days of age. Poult. Sci. 98:1363-1370.
Naber, E. C. 1979. The effect of nutrition on the composition of eggs. Poult. Sci. 58:518-528.
Naber, E. C. and M. W. Squires. 1993. Vitamin profiles of eggs as indicators of nutritional status in the laying hen: diet to egg transfer and commercial flock survey. Poult. Sci. 72:1046-1053.
Nayak, N., R. A. Rajini, J. J. Kirubaharan, S. Ezhilvalavan, and A. R. Sahu. 2018. Effect of In Ovo Feeding of Tryptophan on Post-Hatch Production Performance and Immune Response in Commercial Broilers. Anim. Nutr. Feed Techn. 18:355-366.
Opaliński, S., B. Dolińska, M. Korczyński, K. Chojnacka, Z. Dobrzański, and F. Ryszka. 2012. Effect of iodine-enriched yeast supplementation of diet on performance of laying hens, egg traits, and egg iodine content. Poult. Sci. 91:1627-1632.
Owen, A. L. 2017. Using a direct-fed microbial in broiler breeders to reduce broiler progeny lameness. M.S. Diss. Univ. Georgia. Athens.
Park, S. W., H. Namkung, H. J. Ahn, and I. K. Paik. 2005. Enrichment of vitamins D3, K and iron in eggs of laying hens. Asian-Aust. J. Anim. Sci. 18:226-229.
Parnian, A., B. Navidshad, F. Mirzaei, R. Behmaram, and H. Deldar, H. 2019. Effect of in ovo injection of nicotonic acid, pantothenic acid or folic acid on immune system and growth of broiler chickens. Iran J. Vet. Med. 13:411-420.
Polin, D., W. H. Ott, E. R. Wynosky, and C. C. Porter. 1963. Estimation of thiamine requirement for optimum hatchability from the relationship between dietary and yolk levels of the vitamin. Poult. Sci. 42:925-928.
Pruszynska-Oszmalek, E., P. A. Kolodziejski, K. Stadnicka, M. Sassek, D. Chalupka, B. Kuston, L. Nogowski, M. P., Maiorano, G., Jankowski, J. and M. Bednarczyk. 2015. In ovo injection of prebiotics and synbiotics affects the digestive potency of the pancreas in growing chickens. Poult. Sci. 94:1909-1916.
Ramadan, N. A., A. S. Omar, A. S. A. Bahakaim, and S. M. Osman. 2010. Effect of using different levels of iron with zinc and copper in layer’s diet on egg iron enrichment. Int. J. Poult. Sci. 9:842-850.
Ringrose, R. C., A. G. Manoukas, R. Hinkson, and A. E. Teeri. 1965. The niacin requirement of the hen. Poult. Sci. 44:1053-1065.
Romero-Sanchez, H. 2005. Effect of male broiler breeder feeding programs on growth, reproductive performance, and broiler progeny. PhD Diss. North Carolina State University, Raleigh.
Rosa, A. P., A. Scher, J. O. B. Sorbara, L. S. Boemo, J. Forgiarini, and A. Londero. 2012. Effects of canthaxanthin on the productive and reproductive performance of broiler breeders. Poult. Sci. 91:660-666.
Saino, N., M. Romano, R. P. Ferrari, R. Martinelli, and A. P. Moller. 2005. Stressed mothers lay eggs with high corticosterone levels which produce low quality offspring. J. Exp. Zool. A. Comp. Exp. Biol. 303:998-1006.
Salmanzadeh, M., Y. Ebrahimnezhad, H. A. Shahryar, and R. Beheshti. 2012. The effects of in ovo injection of glucose and magnesium in broiler breeder eggs on hatching traits, performance, carcass characteristics and blood parameters of broiler chickens. Arch. Geflugelkunde. 76:277-84.
Sengor, E., M. Yardimci, S. Cetingul, L. Bayram, H. Sahin, and I. Dogan. 2007. Effects of short chain fatty acid (SCFA) supplementation on performance and egg characteristics of old breeder hens. S. Afr. J. Anim. Sci. 37:158-163.
Shafey, T. M., A. H. Mahmoud, A. A. Alsobayel, and M. A. Abouheif. 2014. Effects of in ovo administration of amino acids on hatchability and performance of meat chickens. South Afr. J. Anim. Sci. 44:123-130.
Scheideler, S. E. 1998. Eggshell calcium effects on egg quality and Ca digestibility in first-or third-cycle laying hens. J. Appl. Poult. Res. 7:69-74.
Slawinska, A., M. Zampiga, F. Sirri, A. Meluzzi, M. Bertocchi, S. Tavaniello, and G. Maiorano. 2020. Impact of galactooligosaccharides delivered in ovo on mitigating negative effects of heat stress on performance and welfare of broilers. Poult. Sci. 99:407-415.
Tabler, G. T., I. L. Berry, and A. M. Mendenhall. 2004. Mortality Patterns Associated with Commercial Broiler Production. In the Poultry Site. 5m Editor. Dec, 13.
Tapeh, R. S., M. Zhandi, M. Zaghari, and A. Akhlaghi. 2017. Effects of guanidinoacetic acid diet supplementation on semen quality and fertility of broiler breeder roosters. Theriogenology. 89:178-182.
Tavaniello, S., R. Mucci, K. Stadnicka, O. Acaye, M. Bednarczyk, and G. Maiorano. 2019. Effect of in ovo administration of different synbiotics on carcass and meat quality traits in broiler chickens. Poult. Sci. 98:464-472.
Toghyani, M., S. Tahmasebi, M. Modaresi, and S. S. Ale Saheb Fosoul. 2019. Effect of arginine and threonine in ovo supplementation on immune responses and some serum biochemical attributes in broiler chickens. Ital. J. Anim. Sci. 18:342-349.
Toosi, S., M. Chamani, M. Shivazad, A. A. Sadeghi, and S. N. Mousavi. 2016. Effects of in ovo injection and inclusion a blend of essential oils and organic acids in high NSPS diets of broiler breeders on performance of them and their offspring. J. Poult. Sci. 53:192-200.
Triques, G. E., A. B. D. Cristo, M. Canevese, P. F. D. S. Marques, A. M. Burin Junior, and J. I. M. Fernandes. 2019. Effect of antioxidant supplementation in diets of roosters during the post-peak phase on reproduction and production characteristics of offspring. Cienc. Anim. Bras. 20:e-43072.
Tsang, C. P. W. 1992. Research note: calcitriol reduces egg breakage. Poult. Sci. 71:215-217.
Ulmer-Franco, A. M., G. M. Fasenko, and E. E. O’Dea Christopher. 2010. Hatching egg characteristics, chick quality, and broiler performance at 2 breeder flock ages and from 3 egg weights. Poult. Sci. 89:2735-2742.
Valle, R. 2008. Estimulando la postura de huevos en nido. Boletín de Servicio. Arbor Acres. May.
Van den Brand, H., M. P. Sosef, A. Lourens, and J. van Harn. 2016. Effects of floor eggs on hatchability and later life performance in broiler chickens. Poult. Sci. 95:1025-1032.
Xie, C., H. A. M. Elwan, S. S. Elnesr, X. Y. Dong, and X. T. Zou. 2019. Effect of iron glycine chelate supplementation on egg quality and egg iron enrichment in laying hens. Poult. Sci. 98:7101-7109
Yair, R., R. Shahar, and Z. Uni. 2015. In ovo feeding with minerals and vitamin D3 improves bone properties in hatchlings and mature broilers. Poult. Sci. 94:2695-2707.
Yoho D. E., J. R. Moyle, A. D. Swaffar, and R. K. Bramwell. 2008. Effect of incubating poor quality broiler breeder hatching eggs on overall hatchability and hatch of fertile. Poult. Sci. 87 (Suppl.1):148.
Youssef, A. W., E. F. El-Daly, N. A. A. El-Azeem, and M. M. El-Monairy. 2013. Effect of sodium formate on laying hen performance, gastrointestinal tract pH and some blood components under heat stress conditions. Asian J. Poult. Sci. 7:17-26.
Yu, L. L., T. Gao, M. M. Zhao, P. A. Lv, L. Zhang, J. L. Li, Y. Jiang, F. Gao, and G. H. Zhou. 2018. Effects of in ovo feeding of L-arginine on breast muscle growth and protein deposition in post-hatch broilers. Animal. 12:2256-2263.
Zhang, H., K. E. C. Elliott, O. A. Durojaye, S. A. Fatemi, M. W. Schilling, and E. D. Peebles. 2019. Effects of in ovo injection of L-ascorbic acid on growth performance, carcass composition, plasma antioxidant capacity, and meat quality in broiler chickens. Poult. Sci. 98:3617-3625.
Zhao, M. M., T. Gao, L. Zhang, J. L. Li, P. A. Lv, L. L. Yu, F. Gao, and G. H. Zhou. 2017. Effects of in ovo feeding of creatine pyruvate on the hatchability, growth performance and energy status in embryos and broiler chickens. Animal. 11:1689-1697.



Very nice article. You are really a very hardworking man. I am following you since I was doing a Master in Poultry Production. Keep it up…. “(Y).”
Like!! Great article post.Really thank you! Really Cool.
Saved as a favorite, I like your website!
Hey There. I discovered your weblog the use of msn. This
is a really neatly written article. I will be sure to bookmark it and
come back to learn extra of your helpful information. Thank you for the post.
I will certainly comeback.
Your place is valueble for me. Thanks!?
Spot on with this write-up, I truly assume this website needs rather more consideration. I抣l in all probability be again to read much more, thanks for that info.
very good put up, i definitely love this website, carry on it
This post presents clear idea in support of the new visitors
of blogging, that truly how to do blogging and site-building.
very good put up, i actually love this website, carry on it
very nice publish, i certainly love this website, carry on it
Thanks so much for providing individuals with an exceptionally special chance to read critical reviews from this blog. It can be very great and also full of fun for me personally and my office mates to search your website no less than thrice in one week to study the latest guides you will have. And of course, I’m just at all times astounded with all the excellent guidelines you serve. Selected 1 facts in this article are particularly the most impressive we have all ever had.
I couldn’t refrain from commenting. Very well written!
My wife and i felt very contented Ervin could complete his studies by way of the precious recommendations he came across while using the blog. It’s not at all simplistic just to find yourself offering facts that many people might have been trying to sell. And we discover we have you to be grateful to for this. The illustrations you’ve made, the straightforward blog menu, the relationships you can make it easier to instill – it’s got many impressive, and it’s really helping our son and our family reckon that the concept is entertaining, and that is unbelievably essential. Many thanks for all the pieces!
I’ve read a few excellent stuff here. Certainly worth bookmarking for revisiting.
I surprise how so much attempt you put to make such a wonderful informative website.
Its like you read my mind! You seem to know a lot about this, like you wrote the book in it or something. I think that you can do with some pics to drive the message home a bit, but instead of that, this is excellent blog. A great read. I will definitely be back.|
Saved as a favorite, I like your web site!|
I believe this is one of the so much important info for me. And i’m happy studying your article. But wanna statement on some normal things, The site taste is ideal, the articles is actually excellent : D. Excellent job, cheers|